|
The Hydrophobic Subtraction Model (HSM) of selectivity has been
developed to quantitatively describe the chromatographic selectivity
of reversed-phase (RP) HPLC columns. Upon characterization of
a given RP stationary phase, the HS model yields quantitative values
for five parameters (H, S*, A, B, and
C) that describe the physico-chemical nature of that
particular phase. Specifically:
- H parameter is a measure of the phase hydrophobicity
- S* is a measure of the resistance of the stationary phase to penetration by a solute molecule
- A is a measure of the hydrogen-bond acidity of the phase
- B is a measure of the hydrogen-bond basicity of the phase
- C is a measure of the interaction of the phase with ionized solute molecules
These parameters, along with the companion parameters that
describe the same characteristics of a given solute (η, σ,
β, α, κ) are related to the retention of that solute (kx)
relative to the retention of a reference compound (in this case,
ethylbenzene - kEB) by the model:
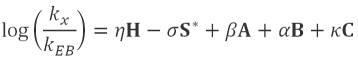
where:
- η parameter is a measure of the solute hydrophobicity
- σ is a measure of the bulkiness of the solute molecule
- β is a measure of the hydrogen-bond basicity of the solute
- α is a measure of the hydrogen-bond acidity of the solute
- κ is a measure of the ionization state of the solute molecule
|
